Transforming Therapeutics
April 17, 2024
UNT researchers are exploring more efficient and sustainable drug discovery techniques and treatments.
BY HEATHER NOEL AND AMANDA FULLER
Miniature human tissues 0.5 to 2 millimeters in diameter have been developed in Moo-Yeal Lee’s Bioprinting Lab at the University of North Texas, which may hold immense potential for the future of health care.
“Our 3D-printed tissues made from pluripotent stem cells serve as promising disease models for screening therapeutic drugs for individual patients,” says Lee, an associate professor of biomedical engineering who has 19 issued and pending patents in South Korea and the U.S.
Lee is one of many professors from UNT’s fast-growing Department of Biomedical Engineering — and across other disciplines — who are seeking new ways to conduct drug discovery research and expanding the foundational science that could lead to new treatments for diseases such as cancer, heart disease and diabetes.
Disease Modeling

Associate professor Moo-Yeal Lee (right) talks with student Sunil Shrestha in his
Bioprinting Lab at UNT. (Photo: Ahna Hubnik/UNT)
Lee’s lab at UNT’s Discovery Park, the largest research park in the North Texas region, has been focused on gene editing of human cells, stem cell differentiation, 3D bioprinting and microfluidic device development to generate human tissues including brain, liver and intestine for disease modeling. By using genetically engineered human cells printed in microfluidic devices, Lee can rapidly generate normal and diseased human tissues, which can be used for modeling critical diseases such as cancer and diabetes, and improving drug screening in the future.
Health care applications for 3D bioprinting have gained momentum in both the clinical and research settings, and Lee’s work in the area could integrate the technology even further. Unlocking the possibilities of 3D bioprinting in health care has been at the core of Lee’s research since he was a postdoctoral scientist experimenting with an early form of the technology in the 2000s. Now, he’s mentoring the next generation of researchers to push boundaries of the field.
Sunil Shrestha is a biomedical engineering doctoral student in Lee’s lab. Shrestha admits at first he felt a bit lost when Lee gave him the freedom to research liver organoids — a miniature liver tissue that can mimic liver function and disease — but he’s learned valuable lessons about planning and conducting his own research.
“I’m not even the same person I was before I started working with Dr. Lee,” Shrestha says. “He encourages us to be openminded. If we want to try something new, he doesn’t restrict us.”
Outside of his academic lab, Lee is an accomplished biomedical entrepreneur. He served as lead scientist for Solidus Biosciences Inc., a bioprinting startup pioneer that grew out of his postdoctoral research, for nearly a decade. Then, in 2017, he founded Farmers Branch-based Bioprinting Laboratories Inc. to commercialize unique tissue culture devices (pillar/perfusion plates) and bioprinted human tissues in the device for conducting organoid-based testing of new drug candidates.
Lee and his research collaborator, Pranav Joshi, are working to generate normal and genetically engineered human liver organoids through microarray 3D bioprinting technology for use in drug discovery. They’ll use the organoids to investigate drug-induced liver injury (DILI) across various ethnic groups.
According to Lee, unexpected adverse drug responses, including DILI, are likely to arise from differences in patient-specific drug metabolism, including individual variability in levels and activities of drug metabolizing enzymes (DMEs) in hepatocytes, which are the cells in the liver that perform a wide range of biotransformation and associated drug toxicity. They’ll use genetically engineered liver organoids to overexpress and downregulate multiple DME genes to simulate different levels of drug metabolism to represent patients from different ethnic groups.
Plant-based Pharmaceuticals
Plant-based medicine has been around for as long as humans have existed, but researchers in UNT’s BioDiscovery Institute are finding new ways to harness plants as manufacturers of medicines.
Assistant professor of chemistry Elizabeth Skellam is leading an interdisciplinary team exploring the potential to develop fungal-derived pharmaceuticals like penicillin in plant hosts for more accessible and environmentally sustainable medicine. The study, supported by a $1.4 million W.M. Keck Foundation grant, is working to establish a new concept for producing valuable fungal products and may ultimately lead to medicines that can be delivered in plant seeds, eliminating downstream processing.
What we’re thinking long-term is that if plants can store medicines in seeds, you
eat the seeds, and the medicine is already contained.
Elizabeth Skellam
“What we’re thinking long-term is that if plants can store medicines in seeds, you eat the seeds, and the medicine is already contained. You don’t have all these factories, you don’t need any chemicals — it’s just there and available,”says Skellam, whose research collaborators include faculty Ana Paula Alonso and Kent Chapman in plant biochemistry and Michael Carroll in economics.
Because the amount of penicillin fungi naturally produce is very small, factory production has been the industry standard since the 1940s, according to Skellam. The specialized facilities require a large amount of energy and equipment, resulting in high levels of chemical waste and irreversible environmental damage. Plants, on the other hand, produce pharmaceuticals via photosynthesis, requiring only sunlight, carbon dioxide, water and mineral nutrients — resources that are easily scalable. Skellam and her team are looking at how to reconstitute the fungal metabolic pathways for penicillin to scale up its natural production.
“Penicillin is one of the handful of fungal-derived drugs where we know exactly where every enzyme in the biosynthetic pathway is located,” Skellam says. “We start with something that we know, then try and replicate it.”
Nanotherapeutic Breakthroughs
Another emerging area revolutionizing therapeutics is nanotechnology, which allows researchers to alter materials on the nanoscale. Brian Meckes, assistant professor of biomedical engineering, is improving nanotherapeutics for treatment of a variety of diseases. His research explores how disease-related physical changes in tissue that accompany diseases like lung cancer or epilepsy impact nanotherapeutic delivery since they have the potential to cause therapies to fail.
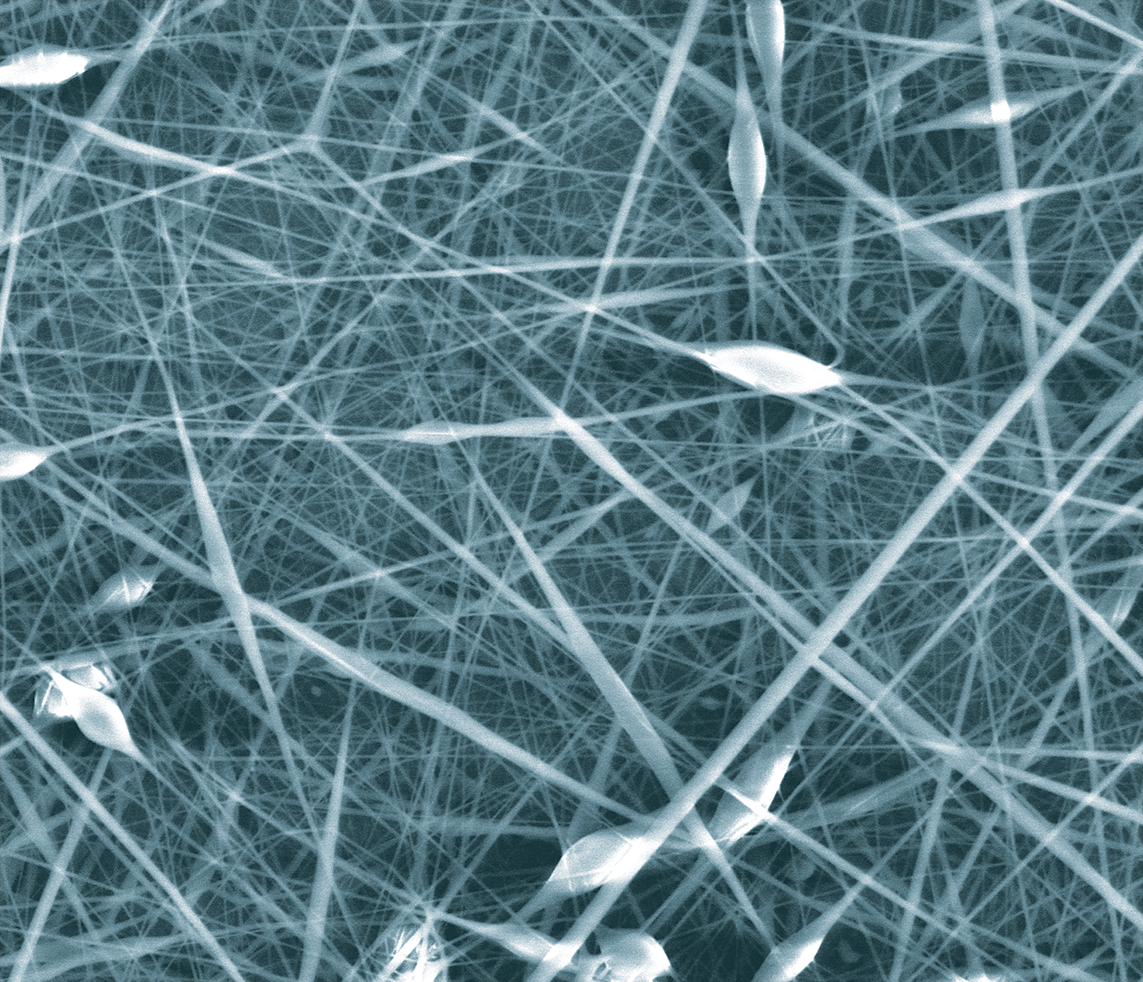
These thin, nanostructures could hold immense potential for breast cancer treatment.
Tissue changes alter how nanotherapeutics interact with cells while creating new opportunities for targeting diseased tissue. By learning more about tissue properties, Meckes can design novel nanoparticles that have improved targeting and trafficking in dysfunctional tissue to deliver an improved treatment outcome.
Neda Habibi, an assistant professor of biomedical engineering, is researching new therapeutic targets that could be used to develop treatments for triple-negative breast cancer (TNBC). Accounting for 10-15% of all breast cancer cases, TNBC is a drug-resistant type of breast cancer that does not express the estrogen receptors, progesterone receptors and human epidermal growth factor receptor 2 (HER2) protein that are seen in other types of cancers.
Rather than focusing on these cell receptors like other cancer treatments do, Habibi and her team of students are investigating an approach using self-assembling peptides — foundational ingredients of nanostructures that aid in more efficient and targeted drug delivery within the body. Specifically, they’re designing peptide substrates of the tyrosine phosphatase enzyme in TNBC that could selectively inhibit TNBC cell growth.
“Our research is looking at ways to make ‘smarter’ nanoparticles that can carry the anti-cancer drug more directly to the tumor cells for release, which could increase the drug’s effectiveness and reduce the impact on normal cells in the body,” Habibi says.
While the fundamental research underway in UNT’s labs is still years away from potential use with patients, it’s impacting the students working on the projects today. Doctoral student Emily Carney (’23) says that because of her experience working in Habibi’s lab, she knows she’d like to establish her own lab someday.
“UNT helped me realize that biomedical engineering is my calling,” Carney says. “This research we’re working on could be a jumping-off point for future scientists to create something that is a very real and helpful treatment for somebody who has breast cancer.”